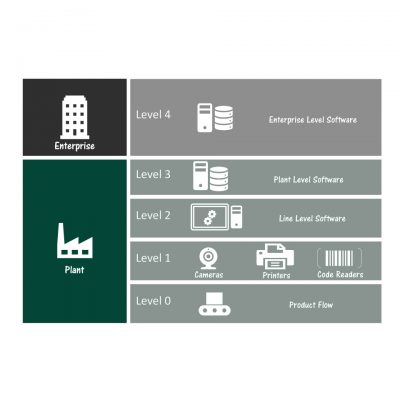
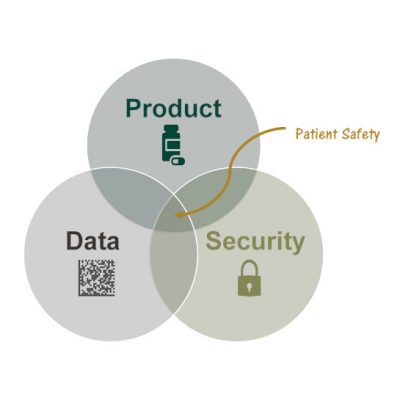
The need to serialize comes from Patient Safety. SAGE understands how Data is used to ensure Supply Chain Security to deliver safe pharmaceutical drug Products to patients.
We offer consulting and engineering services to help you implement end-to-end serialization solutions that are both compliant and cost-effective, including:
- Serialization Roadmap Development
- Compliance Assessment
- Label and Artwork Management
- Line Integration Support
- Recipe Development and Qualification
- Computer Software Validation
- Continuous Improvement / OEE
Our Serialization Strategy
SAGE is vendor-agnostic; however, we have strategically developed relationships with key equipment and software suppliers to better develop solutions for our clients.
We know the Serialization Regulations for each market and how technology can be used effectively to achieve compliance.
SAGE utilizes a four-phase approach to ensure project deliverables are met and full traceability exists from User Requirements Specifications to Start-up:
Plan
- Roadmap Development
- Compliance Assessment
- User Requirements Specification (URS)
- Business Planning
- Change Control
- Vendor Comparison Matrix
- Vendor Selection
Design
- Functional Design Specifications (FDS)
- Detailed Design Specifications (DDS)
- Design Qualification and Review (DQ/DR)
- End-user review and feedback
- Mock-ups
Implement
- Factory Acceptance Testing
- Delivery/Move Plan
- Installation
- Commissioning
- Site Acceptance Testing (SAT)
- Installation Qualification (IQ)
- Operation Qualification (OQ)
Support
- Training
- Start-up/Performance Qualification
- Continuous Improvement
- Line Efficiency Assessment
- Troubleshooting
Key Projects
Equipment & Facility Engineering
End to End
Delivery of Strategic Planning, Project Roadmap, URS, Installation, Commissioning, Qualification, and Start-up for two high-volume solid dose packaging lines
Implementation
Implementation of an enterprise-level serialization solution for a virtual manufacturer with multiple global CMOs
Compliance & Validation
Integration
Retrofit/Upgrade project to enable compliance with global regulations, including software and equipment changes, warehouse/shipping system integration, and production process mapping to build packaging hierarchies (aggregation)
Project Management
Integration
Over 25 serialization/traceability projects for the United States, Russia, China, EU, Saudi Arabia, South Korea and other countries