With a thorough understanding of LEAN principles, SAGE Engineering is able to effectively incorporate the required methodologies as part of continuous improvement projects for companies in the Food and Drug Administration/Health Canada regulated industries.
LEAN is a critical organizational strategy focused on removing waste and increasing process efficiencies to realize time savings, cost savings, and quality improvements. There are a variety of LEAN tools that can be utilized to achieve these outcomes, however, LEAN is more than just the use of tools. LEAN is about the shift in mindset and transformational journey that organizations undertake in pursuit of perfection.
LEAN is built on the foundation of five principles that are employed to improve processes: Define Value, Map Value Stream, Create Flow, Establish Pull, and Pursue Perfection.
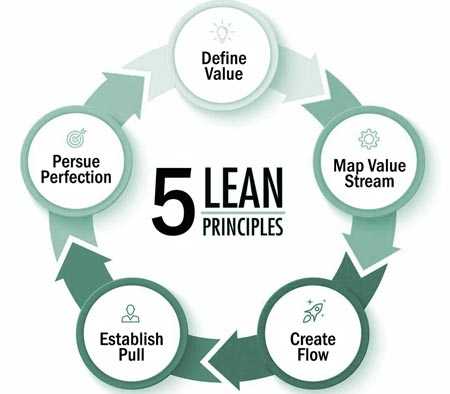
To remove waste and improve efficiencies in a process, value and non-value added tasks must be identified. Value is defined as activities where the product or service is transformed into a state (for the first time) to meet customer requirements. Conversely, non-value added tasks are defined as activities that are not adding value in the creation of products and services and that the customer is not willing to pay for. This is also known as waste.
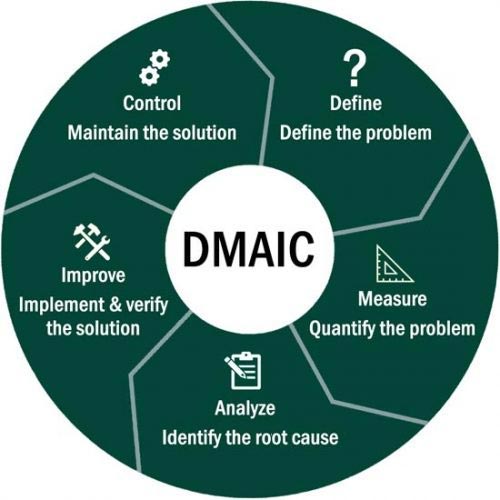
Once waste/non-value-added tasks have been identified, LEAN methodologies can be used to eliminate or reduce them. One such methodology is the Define, Measure, Analyze, Improve and Control (DMAIC) model which is often used to problem solve.
It is important to note that DMAIC can be utilized in a multitude of scenarios where problem-solving and improvements are required, and it is not solely for the purpose of LEAN projects.
Our approach to LEAN projects at SAGE Engineering involves going to the actual workplace, engaging with the people actually doing the work, observing the actual process, collecting the actual data, and defining and understanding the actual value stream/problem/project/etc. We make our recommendations based on performing root cause analysis on the data that we have gathered, and we work with our clients to implement cost saving and time saving opportunities. The Food and Drug Administration and Health Canada regulated industries are laden with tasks that are required to satisfy regulators rather than to directly add value for the customer. SAGE can support and lead projects to analyze existing processes to appropriately identify waste that can be eliminated while retaining tasks that are essential to regulators and customers.
Our services include:
- Process mapping of the current state and the ideal future state by identifying value/non-value added tasks and implementing improvement opportunities
- Reduction of set-up time and change over time using the Kaizen approach
- Reduction and elimination of line stoppages through the application of DMAIC and analysis of problems using tools such as 5 Whys and fishbone
- Organization of the workspace by applying the 5S methodology and engaging and training the affected personnel Implementation of improvements to Autonomous Maintenance and Preventative Maintenance programs
- Implementation of Lean Documentation approach
- Application of tools such as DMAIC, Time and Motion Studies, Root Causes Analysis, etc.
Key Projects
ASEPTIC FILLING LINE
Improvement Initiatives Implementation
Managed the implementation and delivery of improvement initiatives identified using 5 Whys, fishbone analysis, and Single-Minute Exchange of Die (SMED) methods, geared toward reducing set-up and changeover duration on a liquid filling line producing both aseptic and terminal sterilization products.
Project Management
Collaborated with key stakeholders to support the implementation of new tools and optimized procedures. Facilitated meetings with site leadership to present progress and assess/mitigate risks related to proposed improvements.
STERILE DRUG MANUFACTURER
Value Stream Mapping and Capacity Analysis
Performed a detailed study of the production and warehouse processes, including storage space, material and people flow, and capacity analysis for several strategic projects planned for the site as part of a facility optimization project for a sterile drug manufacturer. Created value Stream Maps (VSM’s) for handling of flammable and cold storage raw materials, movement of components (bottles, container closures, labels) and filling, packaging and shipping of drug products. Proposed improvement options to decrease utilization and increase capacity for production and warehouse operations based on process data collected (cycle time, change over time, uptime, downtime, shift working time, and process yield).
SITE UTILITIES
Analyzing, Scoping, and Budgeting
Following the DMAIC process, worked with the site maintenance team to compile a comprehensive list of utilities improvement opportunities. Assessed opportunities and determined high-level steps required to implement improvements. Engaged potential vendors to determine third-party costs associated with potential improvement projects.
Prioritization
Collaborated with client stakeholders to assign metrics and assess identified improvement opportunities. Prioritized projects list for execution, with buy-in from leadership.
ORAL SOLID DOSE PACKAGING
Overall Equipment Effectiveness (OEE) Study
Conducted time and motion studies on primary/secondary filling lines in operation over several days and batches. Acquired stoppage data including type and duration. Reviewed conditions for restart from stoppages with operators and mechanics.
Data Interpretation and Conclusions
Categorized stoppages and performed root cause analysis. Compiled results to identify high-risk stoppage categories/groups, specifically considering frequency and duration. Developed strategies to address OEE deficiencies.
ASSET MANAGEMENT MASTER PLAN
Failure Modes and Effects Analysis (FMEA)
Facilitated FMEA sessions using a Kaizen approach engaging Subject Matter Experts (SME’s) from Maintenance and Engineering to determine failure modes for equipment on sterile and non-sterile liquid filling and packaging equipment, as well as their frequencies and severities. Compiled and assessed results of FMEA as well as applicability to similar filling/packaging lines.
Maintenance Plan and Procedure Optimization
Used FMEA results to build Preventative Maintenance plans and compared with existing plans and procedures. Optimized and implemented new maintenance plans and procedures in collaboration with the client.
DOCUMENTATION OPTIMIZATION
SOPs and Master Batch Records
Worked with client SME’s to group site documents by similarity. Assessed content to identify sources of waste in documented manufacturing, packaging, and maintenance procedures. Combined similar procedures to reduce redundancy and overall number of site-level documents. Collaborated with client stakeholders to review and implement a revised document library.
FACILITY RELOCATION
Process Mapping
Mapped the client’s current processes and used this information to create a conceptual design for the relocation of packing and shipping operations. Developed a model to determine the depletion and replenishment of just-in-time inventory items and determined the number of lines and workstations required to meet output demands.
